Abstract
Introduction: Daratumumab (DARA) is a CD38-targeting IgGκ monoclonal antibody with antimyeloma activity mediated by both on-tumor and immunomodulatory mechanisms of action. In two clinical studies (NCT00574288 [GEN501] and NCT01985126 [SIRIUS]), DARA monotherapy induced rapid, deep, and durable responses with a favorable safety profile in patients with heavily treated, relapsed and refractory multiple myeloma (Lokhorst HM. N Engl J Med 2015;373[13]:1207-1219. Lonial S. Lancet 2016;387[10027]:1551-1560). A combined analysis of patients who received 16 mg/kg DARA in these studies at a median follow-up of 20.7 months was reported previously (Usmani SZ. Blood 2016;128[1]:37-44). Here, we present the final safety and efficacy findings for these patients after median follow-up of approximately 3 years.
Methods: GEN501 was a first-in-human, open-label, phase 1/2 study comprising a dose-escalation phase (Part 1) and a dose-expansion phase (Part 2). The study enrolled patients with MM that had relapsed after or was refractory to ≥2 prior therapies. In Part 2, patients received an initial infusion of 16 mg/kg DARA, which was followed by a 3-week rest period and then by DARA infusions once weekly (QW) for 7 weeks, once every 2 weeks (Q2W) for 14 weeks, and once every 4 weeks (Q4W) thereafter. SIRIUS was an open-label phase 2 study of DARA in patients with MM who had received ≥3 prior therapies, including a proteasome inhibitor (PI) or immunomodulatory drug (IMiD), or who were double refractory to a PI and an IMiD. Patients (n = 106) received 16 mg/kg DARA IV QW for 8 weeks, Q2W for 16 weeks, and Q4W thereafter. Patients treated with 16 mg/kg DARA in GEN501 Part 2 and in SIRIUS were included in this combined analysis. Overall response rate (ORR) was calculated based on computerized algorithm results from both studies.
Results : The combined analysis included 148 patients (42 from GEN501 Part 2 and 106 from SIRIUS) who were treated with 16 mg/kg DARA. Seventy-six percent of patients had received >3 prior therapies. Ninety-one percent of patients were refractory to their last line of therapy, and 87% were refractory to both a PI and an IMiD. In the combined analysis set, the median duration of follow-up was 36.6 (range 0.5-42.3) months.
The most common (≥20%) treatment-emergent adverse events (TEAEs) observed across the 2 studies were unchanged from previous reports: fatigue (42%), nausea (30%), anemia (28%), back pain (27%), cough (26%), upper respiratory tract infection (22%), neutropenia (21%), thrombocytopenia (21%), pyrexia (20%), and nasal congestion (20%). The most common (≥5%) grade 3 or 4 TEAEs were anemia (18%), thrombocytopenia (14%), neutropenia (10%), and hypertension (5%). Infusion-related reactions were observed in 71 (48%) patients and predominantly occurred during the first infusion.
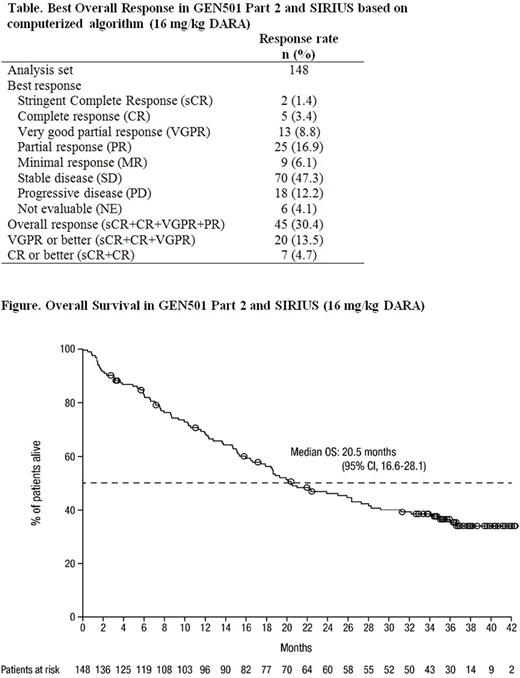
ORR was 30.4% (95% confidence interval [CI], 23.1-38.5), with 20 (13.5%) patients achieving VGPR or better and 7 (4.7%) achieving CR or better (Table). In both studies, deep responses were maintained over time. Among responders, the median duration of response was 8.0 months (95% CI 6.5-14.7), and 19.6% (95% CI 9.0-33.2) of responders remained progression free at 3 years. Median overall survival (OS) was 20.5 months (95% CI 16.6-28.1, Figure), and the 3-year OS rate was 36.5% (95% CI 28.4-44.6). Case reports of patients with prolonged, ongoing responses will be described.
Conclusions: After 3 years of median follow-up, single-agent DARA continues to demonstrate a favorable safety profile with no new safety signals. Deep and durable responses continue to be maintained in a subset of these heavily pretreated patients. With over one-third of patients remaining alive after 3 years of study entry, these findings highlight the activity of single-agent DARA in this heavily pretreated population.
Usmani: Amgen: Consultancy, Honoraria, Speakers Bureau; Novartis: Speakers Bureau; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Research Funding; Array BioPharma: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Weiss: Janssen: Research Funding; Alnylam: Honoraria; Janssen: Honoraria; Prothena: Research Funding; Prothena: Honoraria. Bahlis: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Belch: Amgen, Celgene, Takeda: Honoraria. Lokhorst: OncoImmune: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Voorhees: Novartis: Consultancy; Amgen: Speakers Bureau; Takeda: Consultancy; Oncopeptides: Consultancy; Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy. Richardson: Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Uhlar: Janssen: Employment. Wang: Janssen: Employment. Qi: Janssen: Employment; Johnson & Johnson, LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal